César Pascual-GarcÃa
Luxembourg Institute of Science and Technology, Luxembourg
Title: A redox active self-assembled monolayer both a sensor and actuator
Biography
Biography: César Pascual-GarcÃa
Abstract
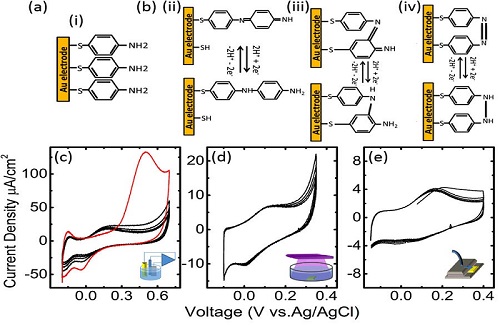
Redox active self-assembled monolayers (SAMs) are emerging materials in the design of key devices like sensors, batteries, or chemical switches. Amino thiol phenol (APT) is a very well studied molecule with a low redox potential, which is able to form SAMs in noble metals thanks to the affinity of its mercapto group and the interactions among the benzene rings. The dissociation constant (pKa) of the amino-group and its interaction with protons has been used to fabricate plasmonic pH sensors associating the molecule to metal nanoparticles which enhance the vibrionic signals that reveal the protonation state of the molecule. Recently, the corresponding concept, namely the modification of the pH through the proton release was proposed by the group of Itamar Willmer. The electrochemical change of the pH was allowed by a composite material which used Au Nanoparticles to increase the surface area to a point where the proton release induced by electrochemical action on the APT was sufficient to achieve a significant modification of the pH. Though, the system showed some signs of electrode degradation, the article proposed a new route to control electrochemical reactions. We studied an alternative to the concept of a nanoparticle concept, by keeping the self-assembled monolayers, which exhibited a better behavior in terms of quasi-reversibility of the proton exchange reactions. We studied different methods of functionalization including the traditional electrochemical polymerization and less common ones like photo- polymerization and plasma polymerization. In addition, we showed that the SAM in addition of sensing it can be used as an effective actuator to control the pH in microfluidic devices. We will propose microfluidic configurations of how use these monolayers and applications to take advantage of the miniaturization possibilities and electrochemical control.